Aetna Approves Coverage for Cleerly's AI Cardiovascular Imaging Analysis
Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...
3 min read
 Cleerly
:
November 11, 2023
Cleerly
:
November 11, 2023

Cleerly, Partners Announce TRANSFORM Trial to Evaluate Personalized Heart Disease Care Strategies at American Heart Association 2023
Data from randomized trial will advance evidence for evaluation and treatment of heart disease by studying actual disease, atherosclerosis
DENVER – November 11, 2023 -- Cleerly, the company working to create a new standard of care for the diagnosis of heart disease, today announced the TRANSFORM trial at the American Heart Association’s (AHA) 2023 Scientific Sessions. TRANSFORM is a randomized trial – sponsored by Cleerly – that aims to enroll 7,500 patients who have pre-diabetes, type 2 diabetes, or metabolic syndrome and have no symptoms of heart disease. TRANSFORM aims to prove that a personalized care strategy based on a Cleerly analysis is better than usual care based on traditional cardiovascular risk factors for the primary prevention of cardiovascular events.
For TRANSFORM, Cleerly is partnering with:
Additionally, the American Heart Association will be providing educational materials (Life’s Essential 8). Cleerly is also a member of the American Heart Association’s Innovators’ Network.
“We are thrilled to start the TRANSFORM trial, which we believe will be a landmark study to demonstrate how a personalized care strategy, fueled by AI, can revolutionize the way we think about prevention of cardiovascular events,” said James K. Min, MD, FACC, FESC, MSCCT, founder and CEO of Cleerly. “By focusing on the individual’s actual coronary artery disease, rather than just risk factors, we aim to prevent heart attacks. I look forward to the progression of this trial with our partners and the results in coming years.”
TRANSFORM will enroll patients at 100-200 sites across the U.S. and treat atherosclerosis in patients with no symptoms or history of heart disease to reduce myocardial infarction (heart attacks). The trial will utilize Cleerly’s investigational plaque staging system in the experimental arm to inform treatment and medication decisions made by providers.
“The TRANSFORM trial brings together the power of AI for staging coronary disease and the well-documented benefits of advanced lipid lowering, anti-thrombotic, anti-inflammatory and cardiometabolic medications to create an individual care plan for each patient at risk for cardiovascular events,” said Udo Hoffmann, MD, MPH, chief scientific officer of Cleerly. “We are excited to partner with academic leaders, professional societies, pharmaceutical companies and healthcare providers to provide scientific proof for a new paradigm in primary prevention of cardiovascular disease that is similar to what has been established for decades in the prevention of cancer.”
Participants will be randomly assigned to the personalized care strategy or to usual care, and all participants will undergo a coronary computed tomography angiography (CCTA) scan. Patients assigned to usual care will be treated based on atherosclerotic cardiovascular disease (ASCVD) risk factors, while those in the personalized care strategy will have an investigational coronary artery disease (CAD) plaque staging system report for both baseline and at 24-months, and CCTA results will be provided to the central cardiologist-led team for discussion and care planning with the patient.
“As study chair and lead investigator, I am excited to embark on the TRANSFORM trial, which we hope will showcase the transformative potential of a personalized care strategy empowered by CCTA scans and AI,” said Deepak L. Bhatt, MD, MPH, FACC, FAHA, FESC, MSCAI, Director of Mount Sinai Fuster Heart Hospital and Dr. Valentin Fuster Professor of Cardiovascular Medicine at Icahn School of Medicine at Mount Sinai. “This trial provides us the opportunity to revolutionize cardiovascular event prevention and ultimately save lives from heart disease. I eagerly anticipate the collaboration with all the valued partners across this trial and the forthcoming results to help us shape the future of cardiovascular care.”
TRANSFORM trial recruitment is scheduled to close in late 2025 and results can be expected in late 2028. For more information, please visit transformtrial.org.
Cleerly is the company on a mission to eliminate heart attacks by creating a new standard of care for the diagnosis of heart disease. Through its AI-empowered solutions, Cleerly supports comprehensive phenotyping of coronary artery disease through its evaluation of advanced non-invasive CT imaging. Cleerly’s approach is grounded in nearly 20 years of science from landmark multi-center clinical trials. Cleerly enhances health literacy for each and every stakeholder in the coronary care pathway. For more information, please visit: www.cleerlyhealth.com.
 Download: JPG |

Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...

Sixth late-breaking presentation from CONFIRM2 Registry demonstrates 12-fold risk gradient based on total plaque burden

New additions streamline data transfer with lesion details, helping make information more accessible for care teams

New alliance combines Cleerly’s AI-enabled approach to identifying early signs of heart disease with Heartbeat Health’s nationwide footprint of...

Cleerly to Present on AI-Enabled Heart Disease Solutions at SCCT 2023Executives to discuss Cleerly’s end-to-end solution for precision heart imaging...
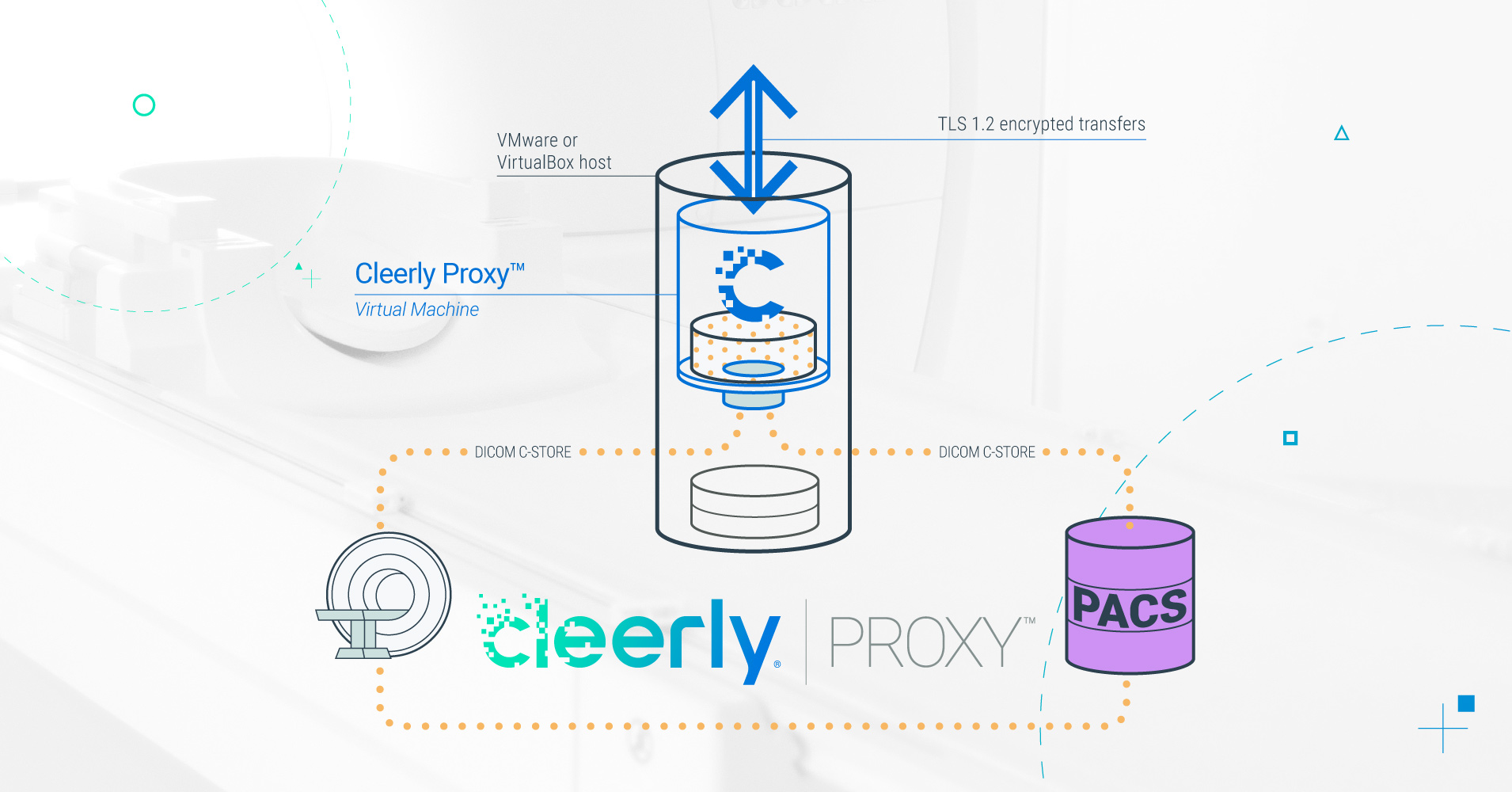
Cleerly introduces Proxy solution to improve heart disease imaging workflowsProxy allows for seamless data transmission and improved AI-based plaque...