Aetna Approves Coverage for Cleerly's AI Cardiovascular Imaging Analysis
Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...
2 min read
 Cleerly
:
January 9, 2024
Cleerly
:
January 9, 2024

FDA cleared, evidence-based AI and machine learning-based algorithm detects heart disease-associated ischemia
DENVER – January 9, 2024 -- Cleerly, the company working to create a new standard of care for the diagnosis of heart disease, today announced the launch of Cleerly ISCHEMIA, which recently received U.S. Food and Drug Administration (FDA) 510(k) medical device clearance. Cleerly ISCHEMIA analysis software is an automated machine learning-based decision support tool, intended as a diagnostic aid for patients undergoing coronary computed tomography angiography (CCTA) analysis using Cleerly LABS software.
Through its AI capability, Cleerly ISCHEMIA determines the likely presence or absence of coronary vessel ischemia based on quantitative measures of atherosclerosis, stenosis, and significant vascular morphology from patients’ CCTA images. When utilized by an interpreting healthcare provider, this software in conjunction with the results of Cleerly LABS provides personalized analysis that may be useful in detecting likely ischemia associated with coronary artery disease (CAD).
“We are pleased to have FDA 510(k) clearance for Cleerly ISCHEMIA and offer the software as part of Cleerly Labs to providers to support their diagnosis of patients across the entire heart disease continuum,” said James K. Min, MD, FACC, FESC, MSCCT, founder and CEO of Cleerly.
Cleerly ISCHEMIA is rooted in over 20 years of science from landmark multi-center trials and is supported by extensive scientific research and validation through comprehensive studies conducted by leading cardiology experts using study data from the CREDENCE and PACIFIC trials.
“Cleerly ISCHEMIA is a true testament to our unwavering commitment to scientific innovation and research-backed solutions,” Dr. Min said. “We are proud to support healthcare professionals personalize patients' heart health journeys. Cleerly ISCHEMIA is a transformative addition poised to reshape the landscape of cardiac care.”
A Category 1 CPT code is available for the work performed in association with Cleerly ISCHEMIA analysis. Cleerly ISCHEMIA analysis is only available by prescription. Interested patients should consult with their physician.
Cleerly is the company on a mission to eliminate heart attacks by creating a new standard of care for heart disease. Through its FDA-cleared solutions driven by artificial intelligence, Cleerly supports comprehensive phenotyping of coronary artery disease, as determined from advanced non-invasive CT imaging. Cleerly’s approach is grounded in science, based on over 10 million images from over 40,000 patients gathered over a 15-year-period in landmark, multi-center clinical trials. Led by a world-class clinical and technical team, Cleerly enhances health literacy for each and every stakeholder in the coronary care pathway. For more information, please visit: www.cleerlyhealth.com.
 Download: JPG |

Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...

Sixth late-breaking presentation from CONFIRM2 Registry demonstrates 12-fold risk gradient based on total plaque burden

New additions streamline data transfer with lesion details, helping make information more accessible for care teams

CREDENCE and PACIFIC-1 study data demonstrate potential power of AI-QCT ISCHEMIA
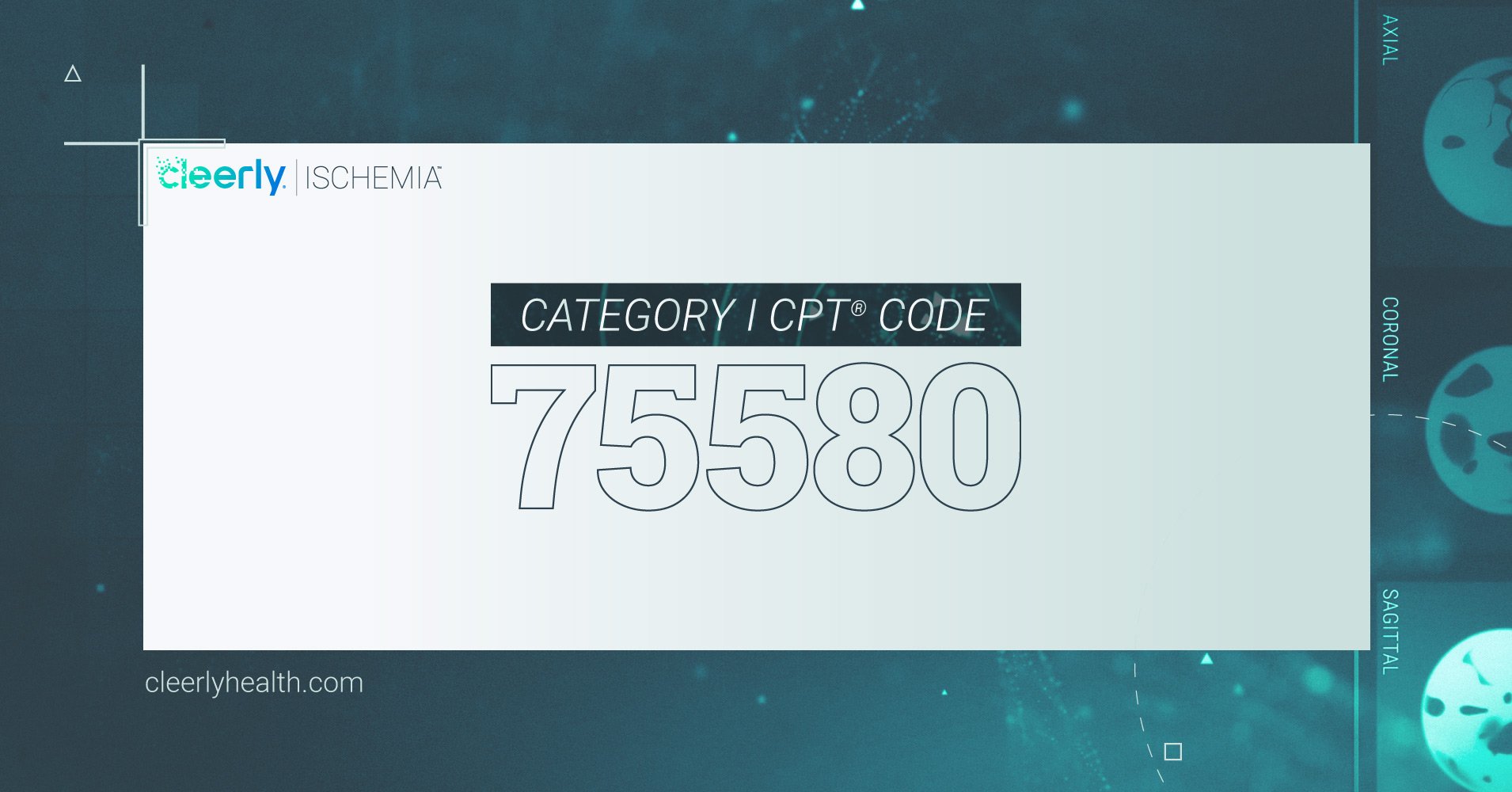
FDA-cleared Cleerly ISCHEMIA will assist healthcare professionals in clinical diagnosis and decision making for coronary artery disease
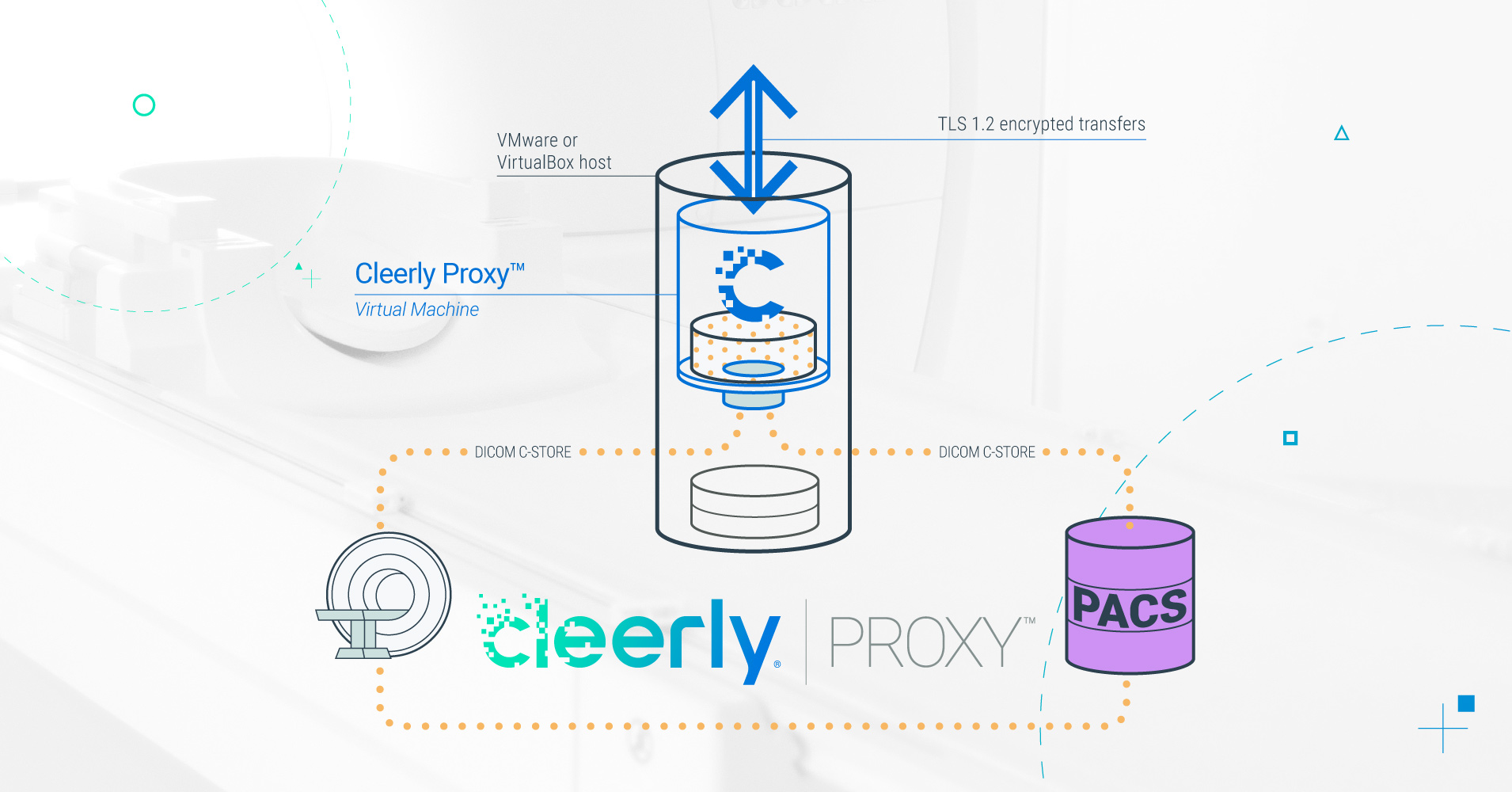
Cleerly introduces Proxy solution to improve heart disease imaging workflowsProxy allows for seamless data transmission and improved AI-based plaque...