Aetna Approves Coverage for Cleerly's AI Cardiovascular Imaging Analysis
Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...
2 min read
 Cleerly
:
December 6, 2022
Cleerly
:
December 6, 2022

Cleerly Hires Udo Hoffmann, MD MPH as Chief Scientific Officer
Cardiovascular clinical trial expert brings AI imaging experience to lead research efforts
New York – December 6, 2022 -- Cleerly, the company creating a new standard of care for heart disease, announced it has appointed Udo Hoffmann, MD MPH, as chief scientific officer. In this role, Dr. Hoffmann will oversee Cleerly’s large-scale research efforts, including its multicenter clinical trials, as well as Cleerly’s relationships with other leading healthcare industry partners.
“It’s with great enthusiasm that we welcome Dr. Hoffmann to Cleerly,” said James K. Min, MD, FACC, FESC, MSCCT, founder and CEO of Cleerly. “Udo has dedicated his professional career to the performance of landmark clinical trials that have fundamentally informed the clinical use of cardiovascular computed tomography (CT), and his scientific abilities are unparalleled. His addition to the Cleerly leadership team will be synergistic in our efforts to develop the scientific evidence and clinical products and services that aim to fundamentally change the way we provide cardiovascular care.”
Prior to joining Cleerly, Dr. Hoffmann was a Professor of Radiology at Harvard Medical School and served as the Chief of the Division of Cardiovascular Imaging at the Department of Radiology at Massachusetts General Hospital (MGH). He also was the Founding Director of the MGH Cardiovascular Imaging Research Center. As a leader in improving cardiovascular prevention and treatment, he was the Principal Investigator of major National Institutes of Health (NIH)-supported clinical trials such as ROMICAT I and II, PROMISE, REPRIEVE and led the CT Imaging in the Framingham Heart Study. His work has resulted in over 600 peer-reviewed publications in such esteemed journals as the New England Journal of Medicine, Journal of the American Medical Association, Lancet and others.
Dr. Hoffmann earned his medical degree and doctorate from the University of Leipzig, Germany, and a Master of Public Health from the Harvard School of Public Health. He completed his residency at Vienna General Hospital.
“Cleerly’s mission to fundamentally change our approach to prevent heart attacks and commitment to generate robust scientific evidence to support this mission is truly inspirational and very much needed,” Dr. Hoffmann said. “I have dedicated my career at Massachusetts General Hospital and Harvard Medical School to using the latest non-invasive imaging technology and artificial intelligence to improve prevention of cardiovascular disease and cancer. I am very excited to join Cleerly to lead upcoming randomized clinical trials and support overall scientific strategy and play a part in taming the world’s #1 killer - coronary atherosclerosis.”
Cleerly is the company creating a new standard of care for heart disease. Through its FDA-cleared, value-based diagnostic solutions driven by machine intelligence, Cleerly enables comprehensive phenotyping of coronary artery disease, as determined from advanced non-invasive CT imaging. Led by a world-class clinical and technical team, Cleerly enhances health literacy for each and every stakeholder in the coronary care pathway. For more information, please visit: https://www.cleerlyhealth.com.
 Download: JPG |

Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...

Sixth late-breaking presentation from CONFIRM2 Registry demonstrates 12-fold risk gradient based on total plaque burden

New additions streamline data transfer with lesion details, helping make information more accessible for care teams
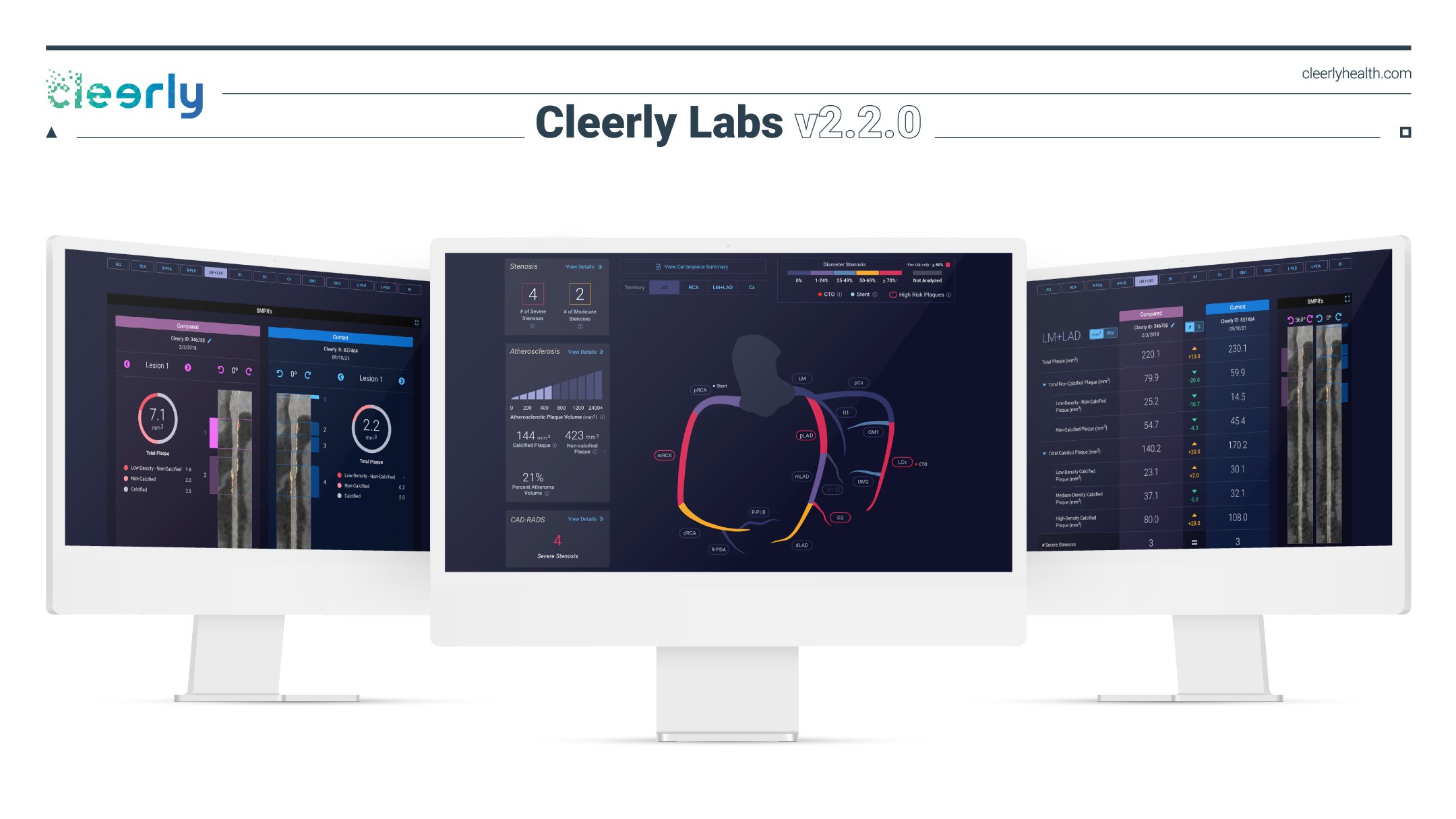
Cleerly 2.2.0 includes UX updates and three high-value tools to better evaluate heart disease. New York, February 8, 2022 -- Cleerly, the company...

Sales strategy expert brings industry success to leverage growth. New York – October 18, 2021 -- Cleerly, the company creating a new standard of care...

Cleerly, the company creating a new standard of care for heart disease, announced today it has expanded its board of directors with three new medical...