Aetna Approves Coverage for Cleerly's AI Cardiovascular Imaging Analysis
Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...

Study Results Demonstrate the Prominent Effects of Cleerly’s Products on Changing Clinical Management for Patients Suspected of Coronary Artery Disease
DENVER – February 8, 2024 -- Cleerly, the company working to create a new standard of care to aid in the diagnosis of heart disease, announced continued strong scientific evidence supporting the clinical utility of its products.
A study published online on January 25, 2024 in the European Heart Journal Cardiovascular Imaging1 demonstrated the independent and strong incremental impact of Cleerly’s products in the prospective multicenter CERTAIN trial of 750 patients conducted at five sites in the U.S. Cleerly’s products were compared to the conventional standard of care interpretation of coronary CT angiography (CCTA) by expert level III imaging physicians. As compared to conventional CCTA interpretation alone, the use of Cleerly’s products led to a significant change in clinical decision making and patient management in more than 57% of patients, and increased physicians’ diagnostic confidence five-fold as compared with standard of care assessment of CCTA.
Use of Cleerly, which performs a comprehensive evaluation of coronary atherosclerosis (plaque), stenosis (narrowing) and ischemia (impaired blood flow), resulted in a 39% change in the assessment of coronary stenosis, a 37% reduction in the need for further invasive and non-invasive testing, and a 28% increase in the use of preventive medications. These results underscore the strong and positive effect of the Cleerly products on clinical utility for patients with suspected coronary artery disease.
“We are excited that our multicenter evidence supports what our rapidly growing customer base has been telling us for some time, namely, that use of Cleerly improves diagnostic accuracy and certainty, while reducing unnecessary downstream resource utilization and potentially harmful invasive testing, and increases the use of preventative medical therapy,” said James P. Earls, MD, Chief Medical Officer of Cleerly.
In all multicenter and single clinical studies performed to date, Cleerly’s products have consistently demonstrated robust clinical utility, including higher diagnostic accuracy and stronger prognostic risk stratification over such historical tests as nuclear SPECT stress testing and FFRCT.2-7
Cleerly is the company on a mission to eliminate heart attacks by creating a new standard of care for heart disease. Through its FDA-cleared solutions driven by artificial intelligence, Cleerly supports comprehensive phenotyping of coronary artery disease, as determined from advanced non-invasive CT imaging. Cleerly’s approach is grounded in science, based on over 10 million images from over 40,000 patients gathered over a 15-year-period in landmark, multicenter clinical trials. Led by a world-class clinical and technical team, Cleerly enhances health literacy for each and every stakeholder in the coronary care pathway. For more information, please visit: www.cleerlyhealth.com.
References:
 Download: JPG |

Major Insurer Joins UnitedHealthcare, Cigna, and Humana in Covering Cleerly's AI-QCT Technology as Industry Momentum Builds with 86+ Million Lives...

Sixth late-breaking presentation from CONFIRM2 Registry demonstrates 12-fold risk gradient based on total plaque burden

New additions streamline data transfer with lesion details, helping make information more accessible for care teams

Cleerly to Present New Clinical Evidence on AI-Enabled CCTA at ACC.23/WCCResearch shows accuracy and effectiveness of Cleerly’s personalized...
New Findings from the CONFIRM2 Registry Reveal Significant Gender Disparities in Coronary Plaque Features and Associated Risks for Major Adverse...
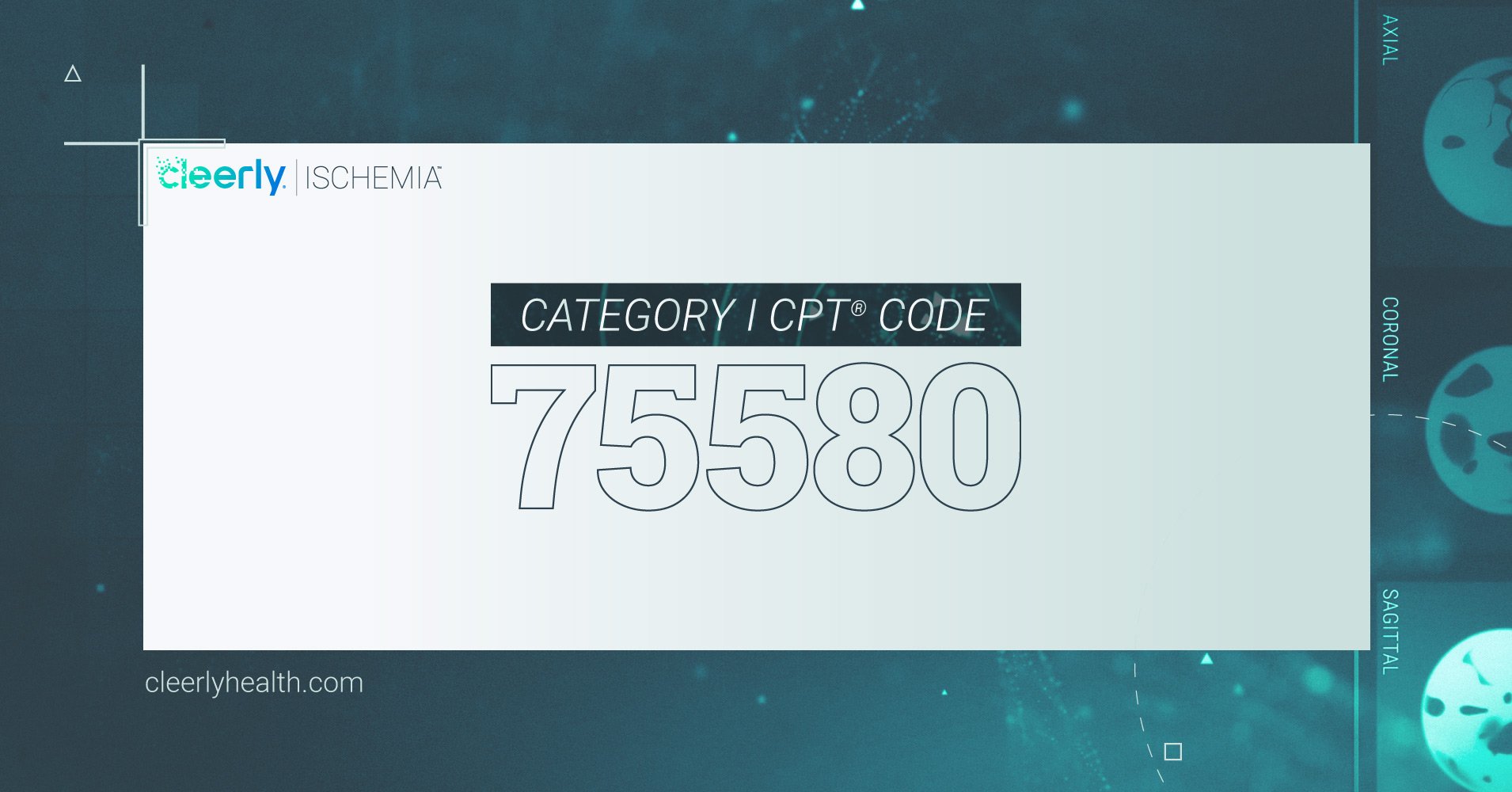
FDA-cleared Cleerly ISCHEMIA will assist healthcare professionals in clinical diagnosis and decision making for coronary artery disease